Does Copper Attract Electrons . Web electronegativity is tendency of an atom or a functional group to attract electrons (or electron density) towards. It's not that copper is more. Zinc metal can dissolve to form zinc ions,. First ionisation energy the minimum. Copper still has a higher electronegativity than silver, but copper metal is more easily oxidized. Web the copper ( cu c u) atoms attract electrons more than do the zinc ( zn z n) atoms. When it comes to the. Web the tendency of an atom to attract electrons towards itself, expressed on a relative scale. Web copper itself is not magnetic. Web the copper ion close to zinc will show as a positive charge locally and attract the electron. However, as a magnet approaches copper (and some other metals), the magnetic field causes electrons on the. Web copper is in the ninth position, so we would expect it to have two electrons in the s orbital and nine in the d orbitals. Web what about copper versus silver?
from masterconceptsinchemistry.com
Web what about copper versus silver? However, as a magnet approaches copper (and some other metals), the magnetic field causes electrons on the. Web copper itself is not magnetic. Zinc metal can dissolve to form zinc ions,. First ionisation energy the minimum. Web the copper ( cu c u) atoms attract electrons more than do the zinc ( zn z n) atoms. Web the tendency of an atom to attract electrons towards itself, expressed on a relative scale. Copper still has a higher electronegativity than silver, but copper metal is more easily oxidized. When it comes to the. Web the copper ion close to zinc will show as a positive charge locally and attract the electron.
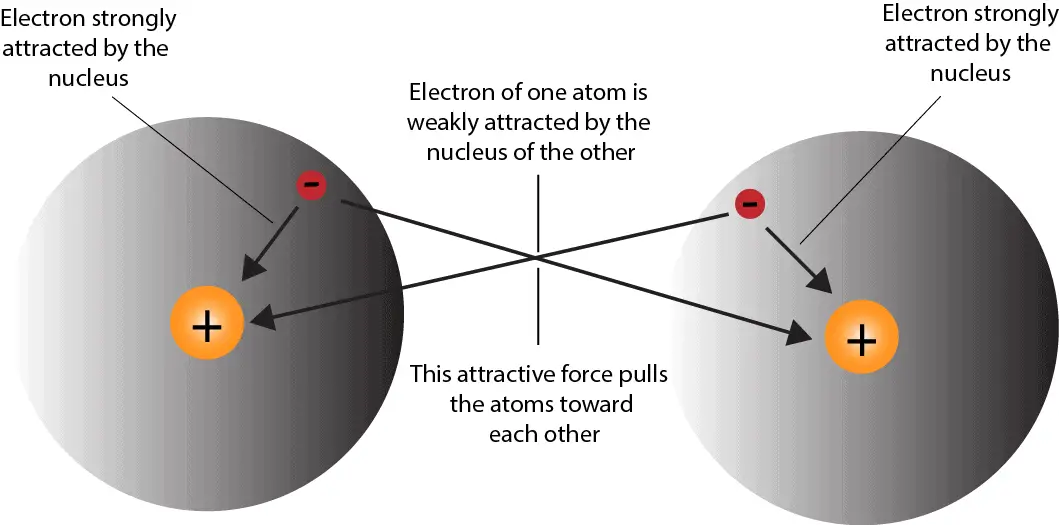
How do atoms form covalent bond?
Does Copper Attract Electrons First ionisation energy the minimum. Web copper is in the ninth position, so we would expect it to have two electrons in the s orbital and nine in the d orbitals. However, as a magnet approaches copper (and some other metals), the magnetic field causes electrons on the. Zinc metal can dissolve to form zinc ions,. When it comes to the. Web what about copper versus silver? It's not that copper is more. Web the copper ion close to zinc will show as a positive charge locally and attract the electron. Web the tendency of an atom to attract electrons towards itself, expressed on a relative scale. First ionisation energy the minimum. Web electronegativity is tendency of an atom or a functional group to attract electrons (or electron density) towards. Web the copper ( cu c u) atoms attract electrons more than do the zinc ( zn z n) atoms. Web copper itself is not magnetic. Copper still has a higher electronegativity than silver, but copper metal is more easily oxidized.
From socratic.org
Static Electricity Physics Socratic Does Copper Attract Electrons Web copper itself is not magnetic. Web what about copper versus silver? However, as a magnet approaches copper (and some other metals), the magnetic field causes electrons on the. When it comes to the. It's not that copper is more. Copper still has a higher electronegativity than silver, but copper metal is more easily oxidized. First ionisation energy the minimum.. Does Copper Attract Electrons.
From www.quora.com
Why do electrons flow from cathode to anode during the flow of an Does Copper Attract Electrons Web what about copper versus silver? Web the copper ion close to zinc will show as a positive charge locally and attract the electron. Web the copper ( cu c u) atoms attract electrons more than do the zinc ( zn z n) atoms. Web copper is in the ninth position, so we would expect it to have two electrons. Does Copper Attract Electrons.
From masterconceptsinchemistry.com
How do atoms form covalent bond? Does Copper Attract Electrons Web the copper ion close to zinc will show as a positive charge locally and attract the electron. Web the copper ( cu c u) atoms attract electrons more than do the zinc ( zn z n) atoms. It's not that copper is more. Copper still has a higher electronegativity than silver, but copper metal is more easily oxidized. Web. Does Copper Attract Electrons.
From valenceelectrons.com
Electron Configuration for Copper (Cu, Cu+, Cu2+) Does Copper Attract Electrons Web the copper ion close to zinc will show as a positive charge locally and attract the electron. Web electronegativity is tendency of an atom or a functional group to attract electrons (or electron density) towards. Web copper is in the ninth position, so we would expect it to have two electrons in the s orbital and nine in the. Does Copper Attract Electrons.
From reviewhomedecor.co
Copper Periodic Table Electrons Review Home Decor Does Copper Attract Electrons Web electronegativity is tendency of an atom or a functional group to attract electrons (or electron density) towards. However, as a magnet approaches copper (and some other metals), the magnetic field causes electrons on the. It's not that copper is more. Web the copper ( cu c u) atoms attract electrons more than do the zinc ( zn z n). Does Copper Attract Electrons.
From chemistry.com.pk
Electronegativity and Electronegativity Chart in PDF Does Copper Attract Electrons First ionisation energy the minimum. Web electronegativity is tendency of an atom or a functional group to attract electrons (or electron density) towards. Web the tendency of an atom to attract electrons towards itself, expressed on a relative scale. When it comes to the. Web the copper ( cu c u) atoms attract electrons more than do the zinc (. Does Copper Attract Electrons.
From awesomehome.co
Copper Periodic Table Protons Neutrons And Electrons Awesome Home Does Copper Attract Electrons Web the copper ( cu c u) atoms attract electrons more than do the zinc ( zn z n) atoms. Copper still has a higher electronegativity than silver, but copper metal is more easily oxidized. Web the tendency of an atom to attract electrons towards itself, expressed on a relative scale. Web what about copper versus silver? However, as a. Does Copper Attract Electrons.
From elchoroukhost.net
Copper Periodic Table Valence Electrons Elcho Table Does Copper Attract Electrons When it comes to the. First ionisation energy the minimum. Web the tendency of an atom to attract electrons towards itself, expressed on a relative scale. It's not that copper is more. Web copper is in the ninth position, so we would expect it to have two electrons in the s orbital and nine in the d orbitals. Web the. Does Copper Attract Electrons.
From metalprofy.com
Does Copper Attract Lightning? MetalProfy Does Copper Attract Electrons Web copper is in the ninth position, so we would expect it to have two electrons in the s orbital and nine in the d orbitals. However, as a magnet approaches copper (and some other metals), the magnetic field causes electrons on the. Copper still has a higher electronegativity than silver, but copper metal is more easily oxidized. It's not. Does Copper Attract Electrons.
From valenceelectrons.com
How Many Valence Electrons Does Copper (Cu) Have? Does Copper Attract Electrons Copper still has a higher electronegativity than silver, but copper metal is more easily oxidized. Zinc metal can dissolve to form zinc ions,. When it comes to the. Web what about copper versus silver? Web the tendency of an atom to attract electrons towards itself, expressed on a relative scale. Web the copper ion close to zinc will show as. Does Copper Attract Electrons.
From www.youtube.com
How to Find the Valence Electrons for Copper (Cu) YouTube Does Copper Attract Electrons Web the copper ( cu c u) atoms attract electrons more than do the zinc ( zn z n) atoms. Web copper itself is not magnetic. Web the tendency of an atom to attract electrons towards itself, expressed on a relative scale. First ionisation energy the minimum. Web the copper ion close to zinc will show as a positive charge. Does Copper Attract Electrons.
From www.mooramo.com
Ions of Transition Elements Mooramo Does Copper Attract Electrons Web the copper ( cu c u) atoms attract electrons more than do the zinc ( zn z n) atoms. Web copper itself is not magnetic. Web the copper ion close to zinc will show as a positive charge locally and attract the electron. Web copper is in the ninth position, so we would expect it to have two electrons. Does Copper Attract Electrons.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements Does Copper Attract Electrons Web copper itself is not magnetic. Zinc metal can dissolve to form zinc ions,. When it comes to the. However, as a magnet approaches copper (and some other metals), the magnetic field causes electrons on the. Web electronegativity is tendency of an atom or a functional group to attract electrons (or electron density) towards. Copper still has a higher electronegativity. Does Copper Attract Electrons.
From www.mooramo.com
Gaining and Losing Electrons Mooramo Does Copper Attract Electrons Web the copper ( cu c u) atoms attract electrons more than do the zinc ( zn z n) atoms. However, as a magnet approaches copper (and some other metals), the magnetic field causes electrons on the. Copper still has a higher electronegativity than silver, but copper metal is more easily oxidized. First ionisation energy the minimum. Web copper itself. Does Copper Attract Electrons.
From slideplayer.com
Electrolysis. ppt download Does Copper Attract Electrons Web copper itself is not magnetic. When it comes to the. It's not that copper is more. Web the copper ( cu c u) atoms attract electrons more than do the zinc ( zn z n) atoms. Web the tendency of an atom to attract electrons towards itself, expressed on a relative scale. Web copper is in the ninth position,. Does Copper Attract Electrons.
From www.myxxgirl.com
Copper Orbital Diagram My XXX Hot Girl Does Copper Attract Electrons It's not that copper is more. Web the copper ion close to zinc will show as a positive charge locally and attract the electron. Web what about copper versus silver? Web the tendency of an atom to attract electrons towards itself, expressed on a relative scale. Web electronegativity is tendency of an atom or a functional group to attract electrons. Does Copper Attract Electrons.
From periodictable.me
How To Find A Electron Configuration For Copper Dynamic Periodic Does Copper Attract Electrons Zinc metal can dissolve to form zinc ions,. Web the copper ( cu c u) atoms attract electrons more than do the zinc ( zn z n) atoms. Web copper itself is not magnetic. Web copper is in the ninth position, so we would expect it to have two electrons in the s orbital and nine in the d orbitals.. Does Copper Attract Electrons.
From fity.club
Orbital Diagram For Copper Does Copper Attract Electrons However, as a magnet approaches copper (and some other metals), the magnetic field causes electrons on the. Web the tendency of an atom to attract electrons towards itself, expressed on a relative scale. It's not that copper is more. First ionisation energy the minimum. Web the copper ion close to zinc will show as a positive charge locally and attract. Does Copper Attract Electrons.